Metabolic Flexibility Begins in the Gut: The Gut–Mitochondria Axis and Human Performance

Short Index
- Foundations of Vitality
Understanding the Gut–Mitochondria Axis as the Master Regulator of Energy and Resilience
- Molecular Drivers of Performance
SCFAs, Butyrate, and Microbial Signaling in Metabolic Flexibility
- The Hidden Limiter
Why Standard Metrics Fail and How Gut Health Determines Recovery and Adaptation
- Precision Optimization Framework
Sequential Strategies to Reduce Inflammation, Enhance Microbiome, Support Mitochondria, and Calibrate Exercise
- The Competitive Edge
Leveraging the Gut–Mitochondria Axis to Amplify All Other Performance and Longevity Interventions
Executive Summary
The gut–mitochondria axis is a bidirectional communication network in which microbial metabolites, particularly butyrate, stimulate mitochondrial biogenesis, enhance oxidative metabolism, and act as epigenetic regulators, while mitochondrial function reciprocally shapes gut barrier integrity and microbiome composition.
Exercise is a key modulator of this axis. Physical activity alters gut microbiota composition, while the microbiome regulates mitochondrial adaptations to training. Short-chain fatty acids (SCFAs) produced during exercise position the gut microbiome as an active regulator of energy production and resilience, rather than a passive bystander.
Compromised gut barrier integrity and chronic low-grade inflammation impair mitochondrial ATP production and shift metabolism toward less efficient pathways. This explains why identical exercise or nutrition protocols can produce vastly different results depending on individual microbiome composition.
Our clinical approach addresses this upstream regulator through a stepwise, personalized framework: reduce inflammation, optimize microbial diversity and SCFA production, support mitochondrial function, and calibrate exercise intensity. By targeting the gut–mitochondria axis as a unified system, patients experience measurable improvements in 8–12 weeks and transformative performance gains in 6–18 months.
In short, optimizing the gut–mitochondria axis is foundational—not optional—for high-level human performance, metabolic resilience, and long-term vitality.
In this article
Here's your private longevity inteliigence report:
Latest Articles
View all
Should You Get a Gut Microbiome Test? A Gastroenterologist’s Evidence-Based Perspective
Last week, a patient arrived in my clinic with results from a direct-to-consumer gut microbiome test. The report labeled her as “deficient” in multiple bacterial species and recommended several probiotic supplements. It included polished graphics, percentile rankings, and a composite “dysbiosis score.”
The challenge was not the presentation—it was the interpretation. The test measured relative bacterial abundance, not microbial function. The reported “deficiencies” reflected comparisons to a reference population of uncertain clinical relevance, rather than actionable biological deficits. Without functional context, these results risked driving unnecessary interventions and expense.
This scenario is increasingly common. As gut microbiome testing becomes more accessible, patients and clinicians alike are confronted with reports that appear precise but often lack clinical clarity. Understanding what these tests measure, their limitations, and when they add real value is essential.
In this article, I will clarify how gut microbiome tests work, what they can and cannot tell us, and how to determine whether testing is appropriate for your health goals.
Executive Summary
- The gut microbiome plays a central role in metabolism, inflammation, immune regulation, and overall healthspan
- Gut microbiome testing analyzes stool samples using DNA sequencing to assess microbial composition and functional capacity
- The two primary testing methods—16S rRNA sequencing and shotgun metagenomic sequencing—offer different levels of insight and clinical utility
- Microbiome tests do not diagnose disease and represent a snapshot in time, not a fixed biological state
- When interpreted appropriately, microbiome testing can enhance precision care; when used in isolation, it can be misleading

The Core Question: What Are We Actually Measuring?
Modern microbiome tests use DNA sequencing to identify microorganisms in your stool. But not all sequencing methods are created equal, and understanding the difference is critical to interpreting what your results actually mean.
16S rRNA Gene Sequencing: The Industry Standard
This is what most consumer tests and many clinical labs use. It works by targeting a specific bacterial gene—the 16S ribosomal RNA gene—that serves as a genetic fingerprint for bacterial identification.
What it does well:
- Identifies bacteria at the genus level (like identifying "Bifidobacterium" but not which specific species)
- Provides a snapshot of overall microbial diversity
- Relatively affordable and fast
- Uses well-established reference databases
Critical limitations:
- Measures relative abundance, not absolute numbers. If you have less Bacteroides, it might mean you actually have fewer Bacteroides, or it could just mean other bacteria increased. The test can't tell the difference.
- Limited species/strain identification. This matters because Lactobacillus rhamnosus GG has clinical evidence, but "Lactobacillus genus" tells us almost nothing actionable.
- No functional information. It's like having a list of kitchen ingredients but no idea what meal you can make. Knowing bacteria are present doesn't tell you what they're producing.
My clinical perspective: 16S sequencing gives you a compositional overview—useful for assessing severe dysbiosis or tracking major shifts after intervention, but often insufficient for precision treatment planning.
Shotgun Metagenomic Sequencing: The Comprehensive Approach
This method sequences all the DNA in your stool sample—not just bacterial genes, but everything present.
What it offers:
- Species and strain-level identification
- Detection of bacteria, archaea, fungi, and viruses
- Functional profiling—analysis of metabolic pathways and gene capacity
- Insight into antimicrobial resistance genes
Why this matters for longevity: Functional data tells us whether your microbiome has the genetic machinery to produce butyrate, metabolize polyphenols, synthesize certain vitamins, or generate inflammatory compounds. This is clinically meaningful information that composition alone cannot provide.
The trade-offs:
- Higher cost
- More complex interpretation
- Requires sophisticated bioinformatics and clinical integration
- Still doesn't tell us what's actually being produced in real-time
When I order shotgun sequencing: For complex cases with treatment-resistant symptoms, metabolic dysfunction despite dietary optimization, or when I need to understand functional capacity before targeted interventions.
What Microbiome Testing Is Not
Let's clear up common confusion:
Traditional stool tests (culture, ova & parasite exams, PCR panels) are not microbiome tests. These look for specific pathogens—they're diagnostic tools for acute infections, not ecosystem profiling. They won't tell you about diversity, composition, or functional capacity.
Blood tests for "leaky gut" or food sensitivities are not microbiome tests. While gut barrier function and immune responses relate to the microbiome, these tests measure different phenomena and shouldn't be conflated with microbiome analysis.
The Technologies You'll Hear About Next
The field is evolving rapidly. Here's what's emerging from research into clinical practice:
Metatranscriptomics: Instead of DNA, this measures RNA. Think of it as the difference between having a recipe book and actually cooking the meal.
Metabolomics: Directly measures the chemical compounds produced by your microbiome—short-chain fatty acids, bile acids, tryptophan metabolites. This is the closest we get to understanding actual physiological impact, and in my view, represents the future of actionable testing.
Mycobiome and virome analysis: Specialized sequencing for fungal communities and bacteriophages. Emerging evidence suggests these play significant roles in health, but clinical applications are still being defined.
These technologies are mostly research-based today but will likely become standard within 5-10 years as costs decrease and interpretation frameworks mature.
My Clinical Framework
When patients ask about microbiome testing, I walk through these questions to maximize the value of their investment:
- What specific question are we trying to answer? "I want to optimize my gut health" is too vague. "I want to understand why I'm still bloated despite FODMAP elimination" is actionable and guides us toward the right test.
- Will the results guide personalized intervention? Testing shines when it reveals specific imbalances that direct targeted treatment—whether that's particular prebiotic fibers, antimicrobial protocols, or dietary modifications you wouldn't have tried otherwise.
- Are we measuring the right thing? Matching the test to your clinical picture maximizes insight. Functional analysis for metabolic concerns, compositional assessment for dysbiosis, targeted sequencing when specific organisms are suspected.
- How will we integrate results with your complete picture? Microbiome data becomes powerful when combined with your symptoms, dietary patterns, medication history, and inflammatory markers—this integration reveals patterns single data points miss.
- What's the intervention and reassessment plan? The greatest value comes from testing, implementing targeted changes, then retesting to confirm improvement—creating a feedback loop that refines your personalized protocol.
The Bottom Line
Microbiome testing can be a valuable clinical tool when:
- Ordered for specific indications, not general curiosity
- Interpreted by someone who understands both the technology and the clinical context
- Used to guide targeted interventions, not generate anxiety about bacterial "deficiencies"
- Followed by appropriate reassessment to measure intervention success.
The microbiome matters immensely for healthspan and longevity. But understanding what to measure, when to measure it, and how to act on results separates useful clinical data from expensive noise.
Dr. Banerjee is a board-certified gastroenterologist with over 15 years of clinical experience, peer-reviewed publications indexed in PubMed, and deep expertise in gut microbiome science. He advises high-achieving individuals and families on precision longevity and healthspan optimization. Expanded clinical analysis is available through the Private Longevity Briefing.
Selected Peer-reviewed evidence
- Chen S, Chen W, Wang X, Liu S. Mendelian randomization analyses support causal relationships between gut microbiome and longevity. J Transl Med. 2024;22(1):1032. Published 2024 Nov 16. doi:10.1186/s12967-024-05823-2
- Zhang G, Lu Y, Wang Z, et al. Causal relationship between gut microbiota and ageing: A multi-omics Mendelian randomization study. Arch Gerontol Geriatr. 2025;131:105765. doi:10.1016/j.archger.2025.105765
- Liu X, Zou L, Nie C, et al. Mendelian randomization analyses reveal causal relationships between the human microbiome and longevity. Sci Rep. 2023;13(1):5127. Published 2023 Mar 29. doi:10.1038/s41598-023-31115-8
- He D, Liu L, Zhang Z, et al. Association between gut microbiota and longevity: a genetic correlation and mendelian randomization study. BMC Microbiol. 2022;22(1):302. Published 2022 Dec 13. doi:10.1186/s12866-022-02703-x
- Porcari S, Mullish BH, Asnicar F, et al. International consensus statement on microbiome testing in clinical practice. Lancet Gastroenterol Hepatol. 2025;10(2):154-167. doi:10.1016/S2468-1253(24)00311-X

Insulin Resistance Starts in Your Gut—Why Your Glucose Meter Isn’t Telling the Full Story
A 47-year-old executive came to my clinic frustrated. Despite cutting sugar, tracking macros, and exercising six days a week, her fasting glucose crept from 92 to 98 mg/dL over two years. Her physician reassured her she was “fine—not even pre-diabetic yet.”
The problem? She wasn’t fine. Microbiome analysis revealed severely depleted butyrate-producing bacteria and an overgrowth of pro-inflammatory taxa. Her insulin resistance had been developing silently for years—we were just looking in the wrong place.
By the time fasting glucose values become abnormal, it’s often too late to catch early dysfunction. Research increasingly shows that insulin resistance begins in the gut, often a decade before traditional blood sugar tests detects it. Understanding this early microbial contribution is key to preventing and reversing metabolic dysfunction.
Executive Summary
- Insulin resistance begins in the gut, often years before glucose becomes abnormal
- Microbial diversity and butyrate-producing bacteria are central to insulin sensitivity
- Short-chain fatty acids act as metabolic regulators, not just digestive byproducts
- Cutting sugar without supporting gut health misses the underlying mechanism
- Early detection through microbiome and insulin assessment enables preventive intervention
- Metabolic resilience—not just lower glucose—should be the goal

Why Your Glucose Meter Tells an Incomplete Story
Insulin sensitivity reflects how efficiently your tissues—muscle, liver, and fat—respond to insulin's signal to take up and use glucose. When sensitivity declines, your pancreas compensates by pumping out more insulin to maintain normal glucose levels.
This means you can have completely normal fasting glucose and HbA1c while your insulin levels are silently climbing—a state called compensatory hyperinsulinemia. This precedes metabolic syndrome and type 2 diabetes by years, sometimes decades.
Here's what most people miss: This early dysfunction doesn't start with excess sugar intake. It begins with gut-derived inflammation, altered microbial metabolite production, and impaired intestinal barrier integrity. Your gut microbiome is an upstream regulator of metabolic health, not a passive bystander.
The Microbiome Signature of Insulin Resistance
Large-scale microbiome analyses consistently show that metabolic health correlates with two key microbial features:
Higher microbial diversity associates with lower insulin resistance and reduced type 2 diabetes risk. A recent JAMA Network Open study identified specific bacterial families—particularly butyrate-producing taxa like Christensenellaceae and Ruminococcaceae—whose presence predicts metabolic resilience.
Microbial composition shifts precede metabolic disease. Before glucose becomes abnormal, insulin-resistant individuals show:
- Depletion of beneficial bacteria (Akkermansia muciniphila, Faecalibacterium prausnitzii, Bifidobacterium species)
- Enrichment of proinflammatory taxa (particularly E. coli and related species)
- Reduced capacity for fiber fermentation and SCFA production
This isn't just correlation. These microbial changes drive the inflammatory and metabolic dysfunction that impairs insulin signaling.
How Your Gut Bacteria Control Glucose Metabolism
The gut microbiome influences insulin sensitivity through multiple interconnected pathways:
Short-Chain Fatty Acid Production
When beneficial bacteria ferment dietary fiber, they produce short-chain fatty acids (SCFAs)—primarily butyrate, acetate, and propionate. These molecules are metabolic regulators, not just byproducts:
- SCFAs stimulate GLP-1 secretion from intestinal cells, enhancing glucose-dependent insulin release and improving postprandial glucose control
- Butyrate activates AMPK signaling in muscle and liver, directly improving insulin sensitivity and metabolic flexibility
- SCFAs strengthen intestinal barrier integrity, reducing endotoxin translocation that triggers systemic inflammation
This positions fiber-fermenting bacteria as functional regulators of your metabolic state. When these bacteria are depleted, SCFA production drops—and insulin sensitivity follows.
Inflammatory Tone and Barrier Function
A compromised intestinal barrier allows bacterial fragments (lipopolysaccharide/LPS) to enter circulation, triggering chronic low-grade inflammation. This "metabolic endotoxemia" impairs insulin receptor signaling in muscle, liver, and fat tissue—creating insulin resistance independent of caloric intake or body weight.
Carbohydrate Processing Efficiency
Multi-omics studies reveal that insulin-resistant individuals have elevated fecal carbohydrates—particularly host-accessible monosaccharides. This indicates inefficient microbial processing: dietary carbohydrates pass through without being converted to beneficial SCFAs.
In contrast, microbiomes with robust fiber-fermenting capacity efficiently convert dietary carbohydrates into metabolic regulators. This is why carbohydrate quality and microbial function matter more than carbohydrate quantity alone.
Gut-Brain and Bile Acid Pathways
Additional mechanisms include gut-brain neuronal signaling that modulates hypothalamic insulin sensitivity and microbial modification of bile acids that influence glucose homeostasis through receptor-mediated pathways. The gut functions as a metabolic command center, not just a digestive tube.
Why Cutting Sugar Isn't Enough
Reducing sugar intake may improve glucose readings short-term, but it doesn't address the biological drivers of insulin resistance. Worse, excessive carbohydrate restriction often reduces dietary fiber intake—inadvertently depleting the butyrate-producing bacteria that protect metabolic health.
I've seen this repeatedly: patients cut carbs to single digits, lose the initial weight, then plateau with persistent inflammation and poor metabolic flexibility. Their microbiome testing reveals the problem: they eliminated fiber along with sugar, collapsing microbial diversity.
The real objective isn't just lower glucose values—it's metabolic resilience: the capacity to manage glucose efficiently across varying dietary, training, and stress conditions. That resilience is built in the gut.
My Clinical Approach
When patients come to me concerned about insulin resistance or early metabolic dysfunction, I start with comprehensive metabolic assessment—looking beyond standard glucose markers to include insulin levels, inflammatory markers, and microbiome function when indicated.
From there, we develop a targeted protocol that addresses the root causes: optimizing fiber intake and microbial diversity, supporting SCFA production, reducing gut-derived inflammation, and implementing lifestyle factors that enhance insulin sensitivity. The specific interventions are personalized based on your unique metabolic profile and microbiome findings.
This systematic approach catches metabolic dysfunction early—before glucose becomes abnormal and tissue damage accumulates.
The Early Detection Advantage
Here's what excites me about this gut-first approach: we can identify and intervene on insulin resistance years before conventional markers flag a problem.
A microbiome depleted in butyrate-producers with elevated inflammatory taxa tells me insulin resistance is developing—even when fasting glucose is 85 mg/dL. This allows for early, targeted intervention when reversal is most achievable.
Compare this to waiting until fasting glucose hits 100 mg/dL or HbA1c reaches 5.7%—at that point, you've already spent years in a pro-inflammatory, insulin-resistant state causing cumulative tissue damage.
The gut microbiome provides a metabolic early-warning system. We just need to listen to it.
The Longevity Perspective
Insulin sensitivity isn't just about avoiding diabetes—it's a central pillar of healthspan. Insulin resistance accelerates aging biology through multiple mechanisms: chronic inflammation, mitochondrial dysfunction, advanced glycation end products, and impaired cellular repair processes.
Maintaining insulin sensitivity as you age requires addressing the biological system that regulates it: your gut microbiome. This means supporting microbial diversity, optimizing fiber fermentation, maintaining barrier integrity, and minimizing inflammatory signals from the gut.
When patients ask me about longevity interventions, metabolic health through microbiome optimization consistently ranks at the top. It's foundational, modifiable, and has cascading benefits across every physiological system.
Dr. Banerjee is a board-certified gastroenterologist with over 15 years of clinical experience, peer-reviewed publications indexed in PubMed, and deep expertise in gut microbiome science. He advises high-achieving individuals and families on precision longevity and healthspan optimization. Expanded clinical analysis is available through the Private Longevity Briefing.
Peer-Reviewed Clinical and Mechanistic Research
- Canfora EE, Meex RCR, Venema K, Blaak EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. 2019;15(5):261-273. doi:10.1038/s41574-019-0156-z
- Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761-1772. doi:10.2337/db06-1491
- Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 2019;68(8):1516-1526. doi:10.1136/gutjnl-2019-318427
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860-867. doi:10.1038/nature05485
- Imdad S, Lim W, Kim JH, Kang C. Intertwined Relationship of Mitochondrial Metabolism, Gut Microbiome and Exercise Potential. Int J Mol Sci. 2022;23(5):2679. Published 2022 Feb 28. doi:10.3390/ijms23052679
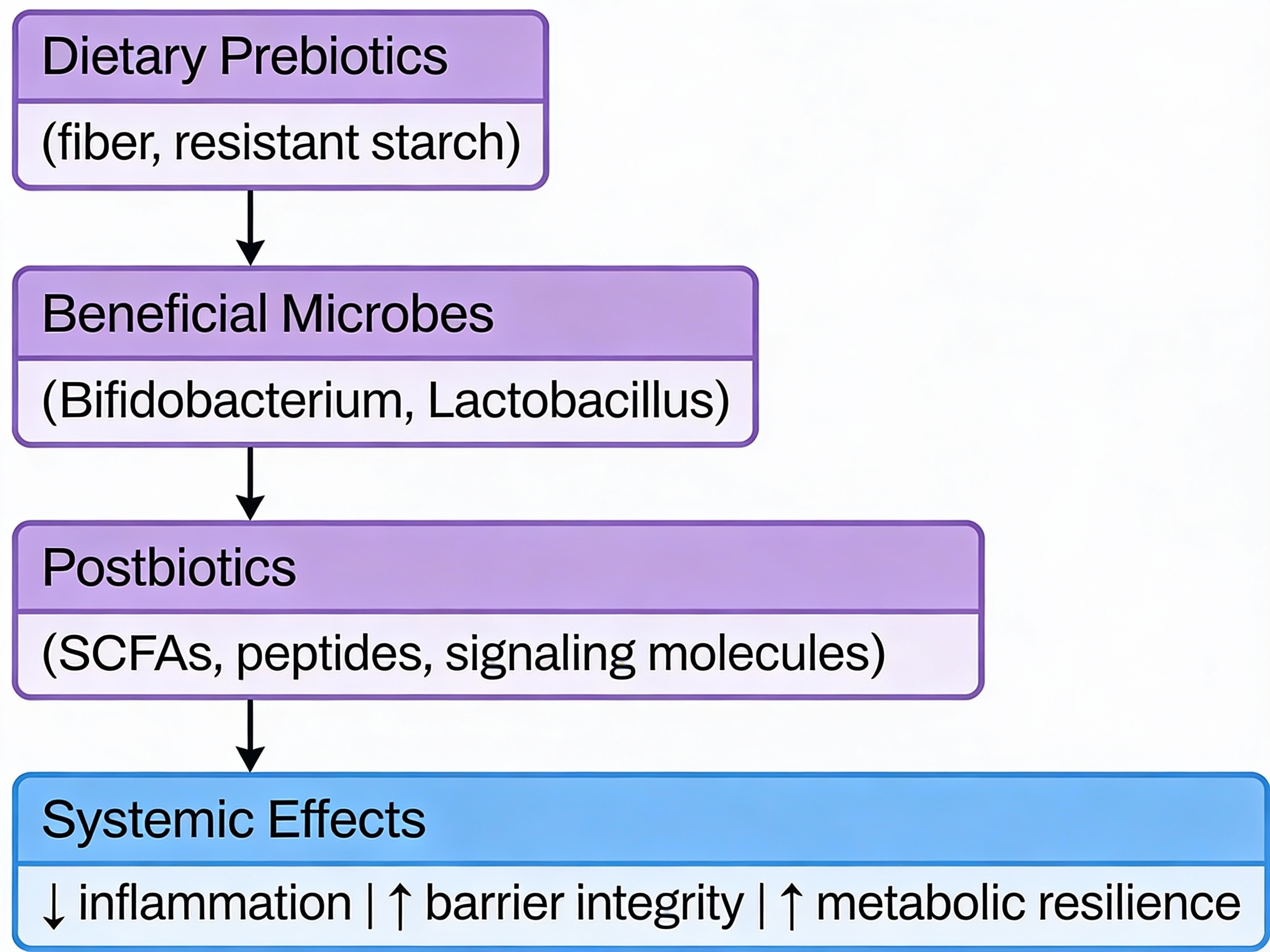
Prebiotics, Probiotics, and Postbiotics Explained: The Gut Health Hierarchy and why Postbiotics Matter More Than You Think
Walk into any health food store, and you'll find hundreds of probiotic supplements making bold claims about gut health. But after 15 years as a board-certified gastroenterologist specializing in longevity medicine, I can tell you: most people are investing in the wrong part of the equation.
The real story of gut health isn't about adding more bacteria—it's about understanding how prebiotics, probiotics, and postbiotics work together, and why that matters for how you age.
Executive Summary
Prebiotics are specialized fibers that feed your beneficial gut bacteria.
Probiotics are live bacteria strains—useful in specific situations, not as daily insurance.
Postbiotics are the bioactive compounds your gut bacteria produce—these are what actually drive the health benefits.
Here's what matters for longevity: A healthy microbiome isn't about the number of bacteria you consume. It's about supporting the ones you have to produce the right metabolic compounds—particularly short-chain fatty acids like butyrate—that reduce inflammation, support metabolism, and influence the biology of aging itself.

Why Your Gut Microbiome Is a Longevity Lever
Your gut microbiome acts as a bridge between what you eat and how your body ages. It influences inflammation, insulin sensitivity, immune function, and even cognitive performance. As we age, microbial diversity typically declines, contributing to chronic inflammation, metabolic dysfunction, and frailty.
The good news? Your microbiome is one of the most modifiable factors in longevity medicine.
The Hierarchy: How I Approach Gut Health in My Clinic
1. Foundation: Prebiotics
Prebiotics are non-digestible fibers that pass through your small intestine intact and reach your colon, where beneficial bacteria ferment them. This fermentation produces short-chain fatty acids (SCFAs)—particularly butyrate—which strengthen your intestinal barrier, reduce systemic inflammation, and support metabolic health.
Key prebiotic types:
- Inulin (chicory root, onions, garlic, leeks, asparagus)
- Resistant starch (green bananas, cooked-and-cooled potatoes/rice, oats)
- Fructooligosaccharides (FOS) and galactooligosaccharides (GOS)
Practical implementation:
- Target 25-35g total fiber daily, including 5-10g prebiotic fiber
- Start low (5g) and increase gradually over 2-3 weeks to minimize gas/bloating
- Diversity matters—rotate different prebiotic sources throughout the week
- Cooked-and-cooled starches are often better tolerated than raw inulin sources initially
Clinical insight: In my practice, consistent prebiotic intake produces more durable microbiome improvements than probiotic supplementation alone. One patient with metabolic syndrome improved her HbA1c by 0.8 points over six months primarily through dietary fiber optimization and polyphenol-rich foods—no probiotics needed.
2. Targeted Tool: Probiotics
Probiotics are live microorganisms that confer health benefits when given in adequate amounts. But here's the critical distinction: probiotics are strain-specific clinical tools, not universal daily supplements.
When I recommend probiotics:
- During or after antibiotic courses (particularly Saccharomyces boulardii or Lactobacillus rhamnosus GG)
- Acute infectious gastroenteritis
- Specific IBS presentations (based on symptom pattern and strain evidence)
- Confirmed dysbiosis with targeted intervention goals
What to look for in quality probiotics:
- Genus, species, and strain clearly listed (e.g., Lactobacillus rhamnosus GG, not just "Lactobacillus")
- CFU count appropriate for indication (typically 1-10 billion)
- Third-party testing for viability
- Enteric coating or acid-resistant capsules for gastric survival
Reality check: Most healthy individuals don't need daily probiotic supplementation. Your microbiome is remarkably stable and self-sustaining when properly nourished. If you're reaching for probiotics as "microbiome insurance" without a specific indication, redirect that investment toward prebiotic-rich foods.
Caution: If you're immunocompromised or have central lines/prosthetic heart valves, discuss probiotic use with your physician first. While generally safe, live bacteria supplementation requires consideration in these contexts.
3. The Real Target: Postbiotics
Here's where it gets interesting. Postbiotics are the metabolic byproducts and cellular components produced by your gut bacteria—and they're what actually deliver the biological benefits we care about.
Key postbiotics include:
- Short-chain fatty acids (butyrate, acetate, propionate)
- Bacteriocins (antimicrobial peptides)
- Exopolysaccharides
- Certain vitamins and enzymes
- Microbial cell wall components
Why postbiotics matter for longevity: These compounds directly interact with your cells to modulate inflammation, support mitochondrial function, strengthen gut barrier integrity, and influence immune tolerance. Butyrate, for example, serves as the primary fuel source for colonocytes while also acting as a histone deacetylase inhibitor—influencing gene expression related to inflammation and aging.
The practical reality: While postbiotic supplements are emerging commercially, the most effective approach is supporting your microbiome's natural postbiotic production through prebiotic fiber, polyphenols, and microbial diversity. When I measure success, I'm looking at inflammatory markers (hs-CRP), metabolic parameters (fasting insulin, HbA1c), and clinical outcomes—not just microbiome composition.
Clinical advantage: Because postbiotics don't rely on live organisms, they're inherently more stable and appropriate for medically complex or immunocompromised patients. As this field matures, targeted postbiotic interventions may become a cornerstone of longevity medicine.
Prebiotics in the Diet: Practical Food Sources
|
Food Source |
Primary Prebiotic Type |
Relative Prebiotic Content |
|---|---|---|
|
Chicory root |
Inulin |
Very high |
|
Garlic |
Inulin, FOS |
High |
|
Onions |
Inulin, FOS |
Moderate–high |
|
Leeks |
Inulin |
Moderate |
|
Asparagus |
Inulin |
Moderate |
|
Oats |
Beta-glucans, resistant starch |
Moderate |
|
Legumes |
Resistant starch, GOS |
Moderate |
|
Green bananas |
Resistant starch |
Moderate |
A diverse intake across these foods supports microbial diversity and sustained postbiotic production.
Putting It Together: A Real-World Protocol
When a patient comes to me wanting to optimize their gut health for longevity, here's my typical approach:
Phase 1: Foundation (Weeks 1-4)
- Dietary assessment and fiber optimization (gradually increasing to 30-35g daily)
- Add diverse prebiotic sources: onions, garlic, asparagus, oats, legumes, cooked-and-cooled starches
- Incorporate polyphenol-rich foods: berries, green tea, extra virgin olive oil, dark chocolate
- Eliminate ultra-processed foods, excess added sugars, and artificial sweeteners
Phase 2: Assessment (Weeks 4-8)
- Monitor for improved regularity, reduced bloating, stable energy
- If significant GI symptoms persist consider comprehensive stool analysis
- Check inflammatory markers (hs-CRP) and metabolic parameters (fasting insulin, lipids)
Phase 3: Targeted Intervention (If Needed)
- Probiotics added only for specific indications: recent antibiotic use, confirmed dysbiosis, symptom-specific IBS treatment
- Duration: typically, 4-12 weeks, not indefinitely
- Reassess and discontinue if no clear benefit
Maintenance:
- Sustainable, fiber-rich dietary pattern (Mediterranean-style is my default)
- Ongoing prebiotic diversity
- Periodic reassessment of inflammatory and metabolic markers
When to Seek Professional Help
Consider working with a gastroenterologist or functional medicine practitioner if you experience:
- Persistent GI symptoms despite dietary optimization (bloating, pain, irregular bowel movements)
- History of recurrent infections or significant antibiotic exposure
- Autoimmune conditions or chronic inflammatory states
- Metabolic syndrome, insulin resistance, or unexplained weight changes
- Cognitive symptoms potentially linked to gut-brain axis dysfunction
The Longevity Perspective
The microbiome research is evolving rapidly, but the fundamentals remain clear: sustainable gut health comes from supporting microbial function and metabolic output, not from indiscriminately adding bacteria.
Think of it this way: prebiotics provide the fuel, probiotics can offer targeted support when needed, and postbiotics deliver the biological effects that influence how you age. The most powerful intervention is usually the simplest; nourishing the ecosystem you already have.
In my clinic, microbiome optimization follows a clear hierarchy:
- Diet-first, fiber- and polyphenol-rich nutrition
- Personalized prebiotic support
- Precision probiotic use when indicated
- Outcome-driven assessment focused on inflammation, metabolism, and resilience
Key Takeaways
- Prebiotics (fiber) are your foundation—they feed beneficial bacteria and drive SCFA production
- Probiotics are targeted tools for specific clinical situations, not daily insurance
- Postbiotics are where the real longevity benefits occur—support their natural production through diet
- Focus on microbial function and metabolic output, not just adding more bacteria
- A whole-food, fiber-rich diet consistently outperforms supplementation alone
Dr. Banerjee is a board-certified gastroenterologist with over 15 years of clinical experience, peer-reviewed publications indexed in PubMed, and deep expertise in gut microbiome science. He advises high-achieving individuals and families on precision longevity and healthspan optimization. Expanded clinical analysis is available through the Private Longevity Briefing.
Selected References
- Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5(4):1417–1435. doi:10.3390/nu5041417.
- Yalçıntaş YM, Bolino MJ, Duman H, et al. Prebiotics: types, selectivity and utilization by gut microbes. Int J Food Sci Nutr. 2025;1–27. doi:10.1080/09637486.2025.2582557.
- Riddle MS, DuPont HL, Connor BA. ACG clinical guideline: acute diarrheal infections in adults. Am J Gastroenterol. 2016;111(5):602–622. doi:10.1038/ajg.2016.126.
- Smolinska S, Popescu FD, Zemelka-Wiacek M. Pre-, pro-, syn- and postbiotics and intestinal integrity. J Clin Med. 2025;14(11):3673. doi:10.3390/jcm14113673.
- Aroniadis OC, Grinspan AM. The gut microbiome: a primer for the clinician. **Am J Gastroenterol.**2024;119(S1):S2–S6. doi:10.14309/ajg.0000000000002583.
- Fontana L, Bermudez-Brito M, Plaza-Diaz J, et al. Sources and evaluation of probiotics. **Br J Nutr.**2013;109(S2):S35–S50. doi:10.1017/S0007114512004011.
- Rana A, Smriti. Probiotics: mechanisms of action and GI health. J Sci Food Agric. 2025. doi:10.1002/jsfa.70275.
- Żółkiewicz J, Marzec A, Ruszczyński M, Feleszko W. Postbiotics—beyond pre- and probiotics. **Nutrients.**2020;12(8):2189. doi:10.3390/nu12082189.
- Thorakkattu P, Khanashyam AC, Shah K, et al. Postbiotics in food and pharmaceuticals. Foods. 2022;11(19):3094. doi:10.3390/foods11193094.
- Wegh CAM, Geerlings SY, Knol J, et al. Postbiotics in early life and beyond. Int J Mol Sci. 2019;20(19):4673. doi:10.3390/ijms20194673.
- Scott E, De Paepe K, Van de Wiele T. Health-modulatory postbiotic biomolecules. **Biomolecules.**2022;12(11):1640. doi:10.3390/biom12111640.
- Nataraj BH, Ali SA, Behare PV, Yadav H. Postbiotics and parabiotics. Microb Cell Fact. 2020;19:168. doi:10.1186/s12934-020-01426-w.
- Gurunathan S, Thangaraj P, Kim JH. Postbiotics as therapeutic agents. Foods. 2023;13(1):89. doi:10.3390/foods13010089.
- Cuevas-González PF, Liceaga AM, Aguilar-Toalá JE. Postbiotics: concepts to applications. **Food Res Int.**2020;136:109502. doi:10.1016/j.foodres.2020.109502.
- Amobonye A, Pillay B, Hlope F, et al. Postbiotics: latest advances. **World J Microbiol Biotechnol.**2025;41(8):293. doi:10.1007/s11274-025-04483-8.
.svg)